 |
Scientific nameChilocorus nigrita (Fabricius) [=Coccinella nigrita Fabricius, Chilocorus nigritus (Fabricius)]
This species is more commonly known in the literature as Chilocorus nigritus, which is incorrect. As per the rules of Zoological Nomenclature, the species name nigrita being a noun, does not change its ending when combined with Chilocorus, a generic name of different gender from the original genus, namely Coccinella (Grenstead, 1951; Booth, 1998). Taxonomic positionColeoptera: Coccinellidae: Chilocorinae: Chilocorini
DescriptionLength 3.2-4.0 mm; width 2.9-3.9 mm. Form subrounded, almost hemispherical and strongly convex. Head dull orange yellow. Pronotum dark pitchy brown to black in middle, paler on sides, anterolateral flaps orange yellow. Elytra black, shiny, with fine punctations. Ventral side including legs
and inner margins of elytral epipleura orange yellow to yellowish brown, outer margins of epipleura pitchy brown to black. Male genitalia and female spermatheca as illustrated. Larva greyish, oval in outline with numerous spiny, branched protuberances on dorsal side.
Chilocorus subindicus Booth, a closely related species belonging to the nigrita-group of species, occurs together with C. nigrita in many parts of southern India, Lakshadweep Islands, etc., but is not as common. It is somewhat smaller in size and can be reliably separated from C. nigrita by the male genitalia. 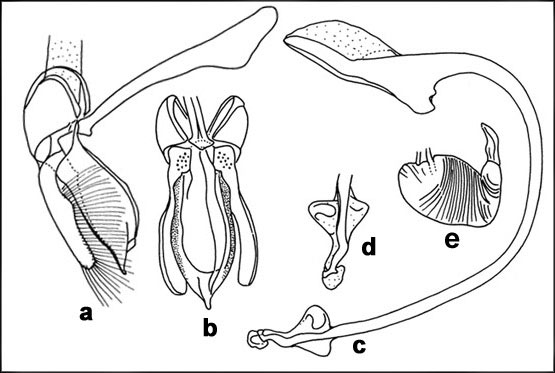 Genitalia of Chilocorus nigrita: a-d. Male genitalia: a. Tegmen, lateral view; b. Tegmen, ventral view; c. sipho; d. siphonal apex; e. female spermatheca ImagesDistributionIndia: Widely distributed (Andhra Pradesh; Assam; Goa; Karnataka; Kerala; Maharashtra;
Orissa; Punjab; Tamil Nadu; Uttar Pradesh). Sri Lanka. Pakistan. Nepal. Myanmar. Malaysia. Chagos Archipelago. Indonesia. Madagascar. Reunion. Mauritius. Seychelles. Togo. Ghana. East Africa. Kenya. Tanzania. South Africa. Brazil.
It is a native of the Indian subcontinent and the most widespread species of this genus. It has been successfully introduced for the biological control of scale insects into, and naturally invaded, many different climatically appropriate regions of the world, including South America and Africa (Samways et al., 1999). Prey / Associated habitatRecorded on numerous hosts, mostly diaspine scales, infesting coffee, arecanut, coconut,
citrus, neem, bamboo, mango, castor, brinjal, sugarcane, Morinda tinctoria, Nerium indicum, Cassia corymbosa, Thevetia, etc.
Specific host records are as follows: HEMIPTERA: Aleyrodidae: Aleurolobus barodensis (Maskell), Aleurodicus dispersus Russell. Aphididae: Aphis sp., Brachycaudus cardui (Linnaeus), Toxoptera citricida (Kirkaldy), indigo aphid. Coccoidea: Aonidiella aurantii (Maskell), Aonidiella citrina (Coquillett), Aonidiella orientalis (Newstead), Aonidiella simplex (Grp. & Charm) (=A. andersoni Laing), Aspidiotus destructor Signoret, A. nerii Bouche, Aspidiotus spp., Asterolecanium miliaris (Boisduval), Asterolecanium sp., Aulacaspis tegalensis (Zehntner), Aulacaspis tubercularis Newstead, Ceroplastes actiniformis Green, Ceroplastodes cajani (Maskell), Chionaspis sp., Chionaspis vitis Green, Chrysomphalus aonidum (Linnaeus) (=C. ficus Ashmead), Chrysomphalus dictyospermi (Morgan), Chrysomphalus pinnulifer diversicolor Green, Coccus colemani Kannan, Coccus hesperidum Linnaeus, Coccus viridis (Green), Diaspis bromeliae (Kerner), Diaspis echinocacti (Bouche), Drepanococcus chiton (Green), Eucalymnatus tessellatus (Signoret), Hemiberlesia lataniae (Signoret), Hsuia sp., Icerya seychellarum (Westwood), Ischnaspis longirostris (Signoret), Lepidosaphes beckii (Newman), Lepidosaphes (as Insulaspis) gloverii (Packard), Lepidosaphes sp., Megapulvinaria (as Pulvinaria) maxima (Green), Melanaspis glomerata (Green), Parasaissetia nigra (Nietner), Parlatoria blanchardi (Targioni Tozzetti), Parlatoria crypta McKenzie, Parlatoria orientalis Ramakrishna Ayyar, Parlatoria ziziphi (Lucas), Phoenicococcus marlatti Cockerell, Pinnaspis aspidistrae (Signoret), Pinnaspis buxi (Bouche), Pinnaspis dysoxyli (Maskell) (as Hemichionaspis minor Maskell), Pinnaspis strachani (Cooley), Palmicultor sp., Planococcus citri (Risso), Prococcus acutissimus (Green), Pseudaulacaspis cockerelli (Cooley), Pseudaulacaspis pentagona (Targioni Tozzetti), Pseudaulacaspis sp., Pseudococcus cryptus Hempel (=P. citriculus Green), Pseudococcus gilbertensis Beardsley, Pseudococcus sp., Pulvinaria psidii Maskell, Pulvinaria polygonata Cockerell, Pulvinaria spp., Diaspidiotus perniciosus (Comstock), Rastrococcus invadens Williams, Saccharicoccus sacchari (Cockerell), Selenaspidus articulatus (Morgan), Tecaspis sp., Vinsonia stellifera (Westwood). Psyllidae: Diaphorina citri Kuwayama, Trioza erytreae (Del Guercio). Seasonal occurrenceCollected almost throughout the year in south India. Mass assemblages of overwintering or
aestivating populations common on banyan trees, particularly during hot months (Tirumala Rao et al., 1954; Ketkar, 1959).
Natural enemiesHomalotylus mexicanus Timberlake, Homalotylus sp. (Hymenoptera: Encyrtidae).
Mass production and field releaseMass production is done on pumpkins infested with Hemiberlesia lataniae. This
species has been used extensively for the control of scale insects on several hosts, particularly citrus, sugarcane, coconut, etc.
Sugarcane: Release in the form of egg pads @ 10 / spot in 100 spots per hectare. Citrus: Ten adults / tree. References
|





