Scientific name
Cheilomenes sexmaculata (Fabricius) (=Chilomenes sexmaculata (Fabricius), Menochilus sexmaculatus (Fabricius), Menochilus
quadriplagiatus (Swartz))
The generic placement of this species has not been conclusively resolved. Recently, Slipinski (2007) has used the name Menochilus sexmaculatus for this species.
Common name
Six-spotted zigzag ladybird
Description
Length 3.3-6.2 mm, width 3.0-5.3 mm. Body outline broadly oval to subrounded, dorsum moderately convex and shiny. Ground colour orange, light red, yellow or pinkish with the following markings in the typical form: head with a black marking in posterior half (Fig. 1); pronotum with a
T-shaped median marking connected to a broad black band along posterior margin; elytra with six black maculae including two zigzag lines and a posterior black spot, sutural line with a narrow to moderately broad black stripe. Ventral side uniformly yellow. Antenna (Fig. 2) short and compact. Prosternal
process (Fig. 3) with a pair of subparallel carinae reaching up to middle. Male genitalia (Figs. 7-9) and female spermatheca (Fig. 6) as illustrated. This is the commonest, yet rampantly misidentified coccinellid of this region due to the occurrence of numerous colour variants. The following variations are frequently seen: (a) Elytra yellowish / pink / orange without any markings except for a black sutural
stripe, (b) elytra and pronotum partially black leaving only the margins, (c) more or less completely
black, and (d) the elytral markings coalesce and form broader bands. The pronotal marking is always constant and can be faintly seen even in completely black forms. All these forms freely intermate and several intermediate forms are found in the field (Subramaniam, 1924). The various colour morphs of this species are frequently misidentified as Micraspis discolor (Fabricius), and Chilocorus nigrita. The larva is dark slaty grey to brown, with yellowish patches. The pupae are yellow with black spots.
Slipinski (2007) has given a detailed description of the genus / species and the larva.
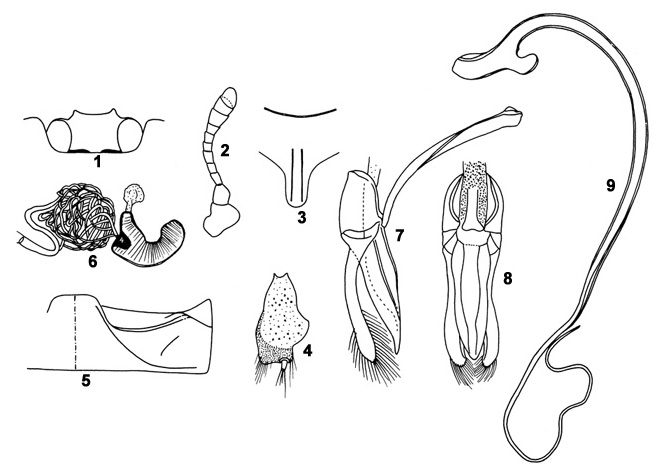 Figs. 1-9. Cheilomenes sexmaculata (F.): 1. Head; 2. Antenna; 3. Prosternal process; 4. Genital plate; 5. First abdominal sternite, postcoxal line; 6. Female spermatheca; 7-9. Male genitalia: 7. Tegmen, lateral view; 8. Tegmen, ventral view; 9. Sipho. Figs. 1-9. Cheilomenes sexmaculata (F.): 1. Head; 2. Antenna; 3. Prosternal process; 4. Genital plate; 5. First abdominal sternite, postcoxal line; 6. Female spermatheca; 7-9. Male genitalia: 7. Tegmen, lateral view; 8. Tegmen, ventral view; 9. Sipho.
Images





 Adult - elytral pattern variations Adult - elytral pattern variations

 Eggs Eggs


 Larva Larva
 Pupae Pupae
Distribution
Almost throughout India and the Oriental region. Iran. Australasia.
Prey / Associated habitat
Aphidophagous, also feeds on psyllids, whiteflies, mealybugs, tingids, leaf- and planthoppers, mites, and early instar lepidopteran larvae. HEMIPTERA: Aleyrodidae:
Aleurodicus dispersus Russell, Aleurolobus barodensis (Maskell), Bemisia tabaci
(Gennadius), Lipaleyrodes euphorbiae David & Subramaniam, Neomaskellia bergii (Signoret), Rusostigma eugeniae (Maskell), Siphoninus phillyreae (Haliday). Aphidoidea: Acyrthosiphon pisum (Harris), Aphis affinis Del Guercio, Aphis craccivora Koch,
A. craccivora pseudoacaciae Takahashi, Aphis cytisorum Hartig, Aphis fabae Scopoli, Aphis gossypii Glover, Aphis longisetosa Basu, Aphis nerii Boyer de Fonscolombe, Aphis pomi De Geer, Aphis spiraecola Patch (as A. citricola van der Goot), Aphis
umbrella (Boerner), Aulacorthum nipponicum (Essig & Kuwana), Aulacorthum solani (Kaltenbach), Brachycaudus helichrysi (Kaltenbach), Brevicoryne brassicae (Linnaeus), Capitophorus himalayensis Ghosh et al., Cavariella simlaensis Chowdhuri et al., Cerataphis brasiliensis (Hempel) (as C. palmae (Ghesquiere)), Ceratovacuna lanigera Zehntner, Cervaphis rappardi indica Basu, Cervaphis schouteniae van der Goot, Chaitophorus himalayensis (Das), Coloradoa rufomaculata (Wilson), Cryptosiphum artemisiae
Buckton, Dreyfusia (as Adelges) knucheli (Schneider-Orelli & Schneider), Greenidea heeri Raychaudhuri et al., Greenideoida ceyloniae van der Goot, Hyadaphis
coriandri (Das), Hyalopterus pruni (Geoffroy), Hyperomyzus lactucae (Linnaeus), Hysteroneura setariae (Thomas), Lipaphis pseudobrassicae (Kaltenbach) (as L. erysimi (Kaltenbach)), Liosomaphis atra Hille Ris Lambers, Macrosiphoniella sanborni (Gillette),
Macrosiphum rosae (Linnaeus), Melanaphis sacchari (Zehntner) (as M. indosacchari (David)), Myzus nicotianae Blackman, Myzus obtusirostris David et al., Myzus persicae (Sulzer), Pemphigus ?napaeus Buckton, Pentalonia nigronervosa Coquerel,
Pineus sp., Rhopalosiphum maidis (Fitch), Rhopalosiphum nymphaeae (Linnaeus), Rhopalosiphum padi (Linnaeus), Rhopalosiphum sp., Schizaphis graminum (Rondani),
Shivaphis celti Das, Sinomegoura citricola (van der Goot), Sitobion akebiae (Shinji),
Sitobion avenae (Fabricius), Sitobion graminis Takahashi, Sitobion ibarae (Matsumura), Sitobion rosaeiformis (Das), Sipha maydis Passerini, Therioaphis trifolii (Monell), Tinocallis kahawaluokalani (Kirkaldy), Toxoptera aurantii (Boyer de
Fonscolombe), Toxoptera citricida (Kirkaldy), Toxoptera odinae (van der Goot),
Tuberculatus nervatus Chakrabarti & Raychaudhuri, Uroleucon compositae (Theobald) (as Dactynotus solidaginis (Fabricius)), Uroleucon carthami (Hille Ris Lambers), Uroleucon (as Dactynotus) formosanus (Takahashi), Uroleucon (as Dactynotus) nigrotuberculatus Olive. Cicadellidae: Amrasca biguttula biguttula (Ishida), Amritodus
atkinsoni (Lethierry), Empoasca kerri Singh-Pruthi, Hishimonus phycitis (Distant),
Empoasca sp., Exitianus indicus (Distant), Deltocephalus sp., Idioscopus clypealis (Lethierry), Nephotettix nigropictus (Stal). Coccoidea: Drepanococcus chiton (Green), Drosicha mangiferae Green, Hemiberlesia lataniae (Signoret), Maconellicoccus hirsutus (Green), Orthezia insignis Browne, Parlatoria blanchardi (Targioni Tozzetti), Phoenicococcus marlatti Cockerell, Pulvinaria psidii Maskell. Delphacidae: Nilaparvata lugens (Stal), Peregrinus maidis (Ashmead), Sogatella
furcifera (Horvath). Lophopidae: Pyrilla perpusilla (Walker). Psyllidae:
Diaphorina citri Kuwayama, Heteropsylla cubana Crawford, Paurocephala psylloptera Crawford, Phylloplecta sp. LEPIDOPTERA: Crambidae: Chilo partellus (Swinhoe). Gelechiidae: Pectinophora gossypiella (Saunders). Lycaenidae: Eggs and/or early instar larvae of Chilades lajus (Stoll). Noctuidae: Earias spp., Helicoverpa armigera (Huebner), Spodoptera litura (Fabricius). Papilionidae: Papilio demoleus Linnaeus.
Tortricidae: Cydia leucostoma Meyrick. Xylorictidae: Opisina arenosella Walker. DIPTERA: Anthomyiidae: Atherigona soccata Rondani. Cecidomyiidae:
Dasyneura lini Barnes. Tephritidae: Acanthiophilus helianthi (Rossi). ACARI:
Tetranychidae: Oligonychus coffeae (Nietner) on tea, Oligonychus mangiferus (Rahman & Sapra), Tetranychus urticae Koch, Tetranychus sp. (ludeni-group). Smartweed (Polygonum hydropiper) and sunnhemp (Crotalaria juncea) are reported to harbour the beetles in large numbers. Collected on maize, sorghum, rice, finger millet, cowpea, gliricidia, cotton, bhendi, brinjal, cabbage, cauliflower, groundnut, lucerne, and several other plants.
Seasonal occurrence
Active almost throughout the year in several parts of India, with many generations.
Natural enemies
Hymenoptera: Braconidae: Dinocampus coccinellae (Schrank); Proctotrupidae: Nothoserphus mirabilis Brues; Encyrtidae: Homalotylus eytelweinii Ratzeburg, Homalotylus flaminius (Dalman), Homalotylus terminalis (Say); Eulophidae: Oomyzus scaposus (Thomson) (= Tetrastichus coccinellae Kurdjumov), Pediobius foveolatus (Crawford), Tetrastichus sexmaculatus Kurian, Tetrastichus sp.; Hemiptera: Pentatomidae: Eocanthecona furcellata (Wolff); Acari: Coccipolipidae: Coccipolipus sp.; Nematoda: Allantonematidae: Parasitylenchus coccinellae Iperti & Waerebeke; Bacteria: Wolbachia sp.



 Larva, pupa, and adult of Dinocampus coccinellae (Schrank), a common parasitoid of lady beetles
Larva, pupa, and adult of Dinocampus coccinellae (Schrank), a common parasitoid of lady beetles
References
- Agarwala, B.K. & Yasuda, H. 2000. Competitive ability of ladybird predators of
aphids: A review of Cheilomenes sexmaculata (Fabr.) (Coleoptera: Coccinellidae) with a worldwide
checklist of preys. Journal of Aphidology, 14: 1-20.
- Booth, R.G., Cox, M.L. & Madge, R.B. 1990. IIE Guides to Insects of Importance to Man. 3. Coleoptera. CAB International, Wallingford, UK, 384 p.
- Pope, R.D. 1989. A revision of the Australian Coccinellidae (Coleoptera). Part I. Subfamily Coccinellinae. Invertebrate Taxonomy, 3 (1988): 633-735.
- Puttarudriah, M. & Channabasavanna, G.P. 1953. Beneficial coccinellids of Mysore-I. Indian
Journal of Entomology 15: 87-96.
- Puttarudriah, M. & Channabasavanna, G.P. 1956. Some beneficial coccinellids of Mysore. Journal of the Bombay Natural History Society 54: 156-159.
- Rahman, K.A. 1940. Important insect predators of India. Proceedings of the Indian Academy of Sciences (B) 12: 67-74.
- Sasaji, H. 1971. Fauna Japonica. Coccinellidae (Insecta: Coleoptera). Academic Press of Japan. 340 p.
- Sasaji, H. & Akamatsu, M. 1979. Reproductive continuity and genetic relationships in the forms of the genus Menochilus (Coleoptera: Coccinellidae). Memoirs of the Faculty of Education, Fukui University, Series II (Natural Sciences) 29: 1-18.
- Slipinski, A. 2007. Australian ladybird beetles (Coleoptera: Coccinellidae) their biology and Classification. Australian Biological Resources Study, Canberra, 306 p.
- Stebbing, E.P. 1903. Coleoptera 2. Notes upon the known predaceous Coccinellidae of the Indian region, Part I. Indian Museum Notes, VI(1): 47-62.
- Subramaniam, T.V. 1924. A note on colour variations in a common ladybird beetle, Chilomenes sexmaculata Fb. In: Report of the Proceedings of the Fifth Entomological Meeting (Ed. T.B. Fletcher) held at Pusa.
|