Scientific name
Brumoides suturalis (Fabricius) (=Brumus suturalis (Fabricius))
Taxonomic position
Coleoptera: Coccinellidae: Chilocorinae: Chilocorini
Diagnosis
Length 4.0 mm, width 2.7 mm. Form oval, dorsum convex. Head and pronotum orange yellow. Scutellum black. Elytra satiny white to creamy yellow, with three black stripes (vittae), one on each elytron in a mid-dorsal position not extending to apex and one along sutural line nearly extending to apex, apical portion yellowish to reddish brown. Anterior clypeal margin (Fig. 1) medially emarginate. Antenna (Fig. 2) eight-segmented. Maxillary palpi (Fig. 3) with terminal segment subcylindrical, apical margin obliquely transverse. Postcoxal line on abdominal ventrite 1 (Fig. 4) complete. Last visible abdominal segment with posterior margin emarginate in male and narrowly rounded in female. Tarsal claw (Fig. 5) simple. Male genitalia (Figs. 6, 7) as illustrated. Larva slaty grey, with prominent spiny protuberances on dorsal side.
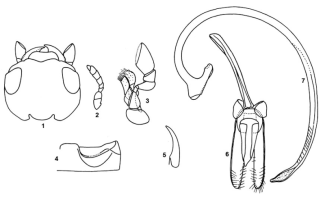 Figs. 1-7. Brumoides suturalis: 1. Head; 2. Antenna; 3. Maxilla; 4. Postcoxal line on abdominal ventrite I; 5. Tarsal claw; Figs. 1-7. Brumoides suturalis: 1. Head; 2. Antenna; 3. Maxilla; 4. Postcoxal line on abdominal ventrite I; 5. Tarsal claw;
6-7. Male genitalia; 6. Tegmen, ventral view; 7. Sipho.Images

 Larva Larva
 Larvae feeding on scales Larvae feeding on scales


 Adult of B. suturalis Adult of B. suturalis
Distribution
Widespread almost throughout India (Andhra Pradesh; Goa; Jammu & Kashmir; Karnataka; Kerala; Manipur; Punjab; Tamil Nadu; Uttar Pradesh; West Bengal). Nepal. Bhutan. Sri Lanka.
Prey / Associated habitat
This species departs from the normal food habits of the other genera of Chilocorini, which are mainly scale feeders. It is more polyphagous and largely predatory on aphids, whiteflies, psyllids, scales, mealybugs and mites. Gorham (1894) reported its feeding on pollen of grasses.
Specific host records are as follows: HEMIPTERA: Aleyrodidae: Aleurocanthus woglumi Ashby,
Aleurolobus barodensis (Maskell), Aleurolobus citrifolii Corbett, Bemisia tabaci (Gennadius), Dialeurodes elongata Dozier, Dialeurodes citri (Ashmead), Neomaskellia andropogonis Corbett, Trialeurodes ricini (Misra). Aphidoidea: Acyrthosiphon pisum (Harris), Adelges sp., Aphis affinis Del Guercio, Aphis craccivora Koch, Aphis
fabae Scopoli, Aphis gossypii Glover, Aphis nerii Boyer de Fonscolombe, Asiphonella
cynodonti (Das), Brachycaudus pruni (Koch), Dactynotus carthami (Hille Ris Lambers), Uroleucon compositae (Theobald), Hyalopterus atriplicis (Linnaeus), Hyadaphis coriandri (Das), Lipaphis pseudobrassicae (Kaltenbach) (as L. erysimi (Kaltenbach)), Myzus
persicae (Sulzer), Pemphigus cynodonti Das, Rhopalosiphum nymphaeae (Linnaeus), Therioaphis trifolii (Monell), Toxoptera aurantii (Boyer de Fonscolombe). Cicadellidae: mpoasca kerri Singh-Pruthi, Hishimonus phycitis (Distant), Nephotettix virescens (Distant), Orosius albicinctus Distant, groundnut jassids. Coccoidea: Coccidohystrix
insolita (Green), Diaspidiotus perniciosus (Comstock) (=Quadraspidiotus perniciosus (Comstock)), Ferrisia virgata (Cockerell), Phenacoccus sp., Pseudococcus cryptus Hempel (as P. citriculus Green), Pseudococcus saccharicola Takahashi, Pseudococcus sp., Maconellicoccus hirsutus (Green), Melanaspis glomerata (Green), Nipaecoccus viridis (Newstead), Trabutina (as Naiacoccus) sp. Delphacidae: Nilaparvata lugens (Stal), Sogatella furcifera (Horvath). Lophopidae: Pyrilla perpusilla (Walker), Pyrilla spp. Psyllidae: Diaphorina citri Kuwayama, Psylla isitis Cotes.
LEPIDOPTERA: Crambidae: Chilo partellus (Swinhoe). Pyralidae: eggs of Scirpophaga excerptalis (Walker). Noctuidae: Earias vittella (Fabricius), E. insulana (Boisduval), early instar larvae of Helicoverpa armigera (Huebner). DIPTERA: Anthomyiidae:
Atherigona soccata Rondani. ACARI: Tetranychidae: Oligonychus coffeae (Nietner) (as Tetranychus bioculatus Wood-Mason), Tetranychus urticae Koch (as T. neocaledonicus Andre).
Collected on sugarcane, maize, brinjal, rice, wheat, cotton, bhendi, cowpea, groundnut,
sunflower, safflower, sesamum, coconut, pigeonpea, castor, sorghum, cabbage, indigo, peach, cumin, mustard, lucerne, tobacco, sandal, pongamia, crotalaria, rice bean, coriander, soyabean, bittergourd, Japanese mint, Citrus limon, Euryale ferox, Thevetia neriifolia, Triumphetta, Achyranthes spera, Tribulus terrestris, Abutilon indicum, Lathyrus sativus, and Sida spinosa.
Seasonal occurrence
It is active throughout the year, except extreme winter (Chandrababu et al., 1997). Abundant on brinjal mealybug infestations almost throughout the year (Puttarudriah &
Channabasavanna, 1957). It can be mass produced in the laboratory on Ferrisia virgata on potato sprouts.
Natural enemy
Homalotylus flaminius (Dalman) (Hymenoptera: Encyrtidae).
References
- Chandrababu, A., Gautam, R.D. & Garg, A.K. 1996. Economics of mass production of predatory beetle, Brumoides suturalis (Fabricius). New Botanist, 23: 73-81.
- Chandrababu, A., Gautam, R.D. & Garg, A.K. 1997. Effect of temperature and relative humidity on the development of predatory beetle, Brumoides suturalis (Fabricius). Annals of Plant Protection Sciences, 5: 142-145.
- Chandrababu, A., Gautam, R.D. & Garg, A.K. 1997. Residual toxicity of some insecticides against the predatory beetle, Brumoides suturalis (Fabricius). Annals of Plant Protection Sciences, 5: 131-136.
- Chandrababu, A., Gautam, R.D. & Garg, A.K. 1997. Feeding potential and associated behaviour of predatory beetle, Brumoides suturalis (Fabricius). Annals of Plant Protection Sciences, 5: 53-60.
- Chandrababu, A., Gautam, R.D. & Garg, A.K. 1999. Biology of ladybird beetle, Brumoides
suturalis (Fabricius) on aphid and mealybugs. Annals of Plant Protection Sciences, 7:
13-18.
- Kapur, A.P. 1942. Bionomics of some Coccinellidae predaceous on aphids and coccids in North India. Indian Journal of Entomology, 4: 49-66.
- Puttarudriah, M. & Channabasavanna, G.P. 1953. Beneficial coccinellids of Mysore-I. Indian
Journal of Entomology 15: 87-96.
- Puttarudriah, M. & Channabasavanna, G.P. 1956. Some beneficial coccinellids of Mysore. Journal of the Bombay Natural History Society 54: 156-159.
- Stebbing, E.P. 1903. Coleoptera 2. Notes upon the known predaceous Coccinellidae of the Indian region, Part I. Indian Museum Notes VI(1): 47-62.
|