Scientific name
Cryptolaemus montrouzieri Mulsant, 1853: 268
Cryptolaemus montrouzieri montrouzieri Booth & Pope, 1986: 706
Common name
Australian ladybird, mealybug destroyer
Taxonomic position
Coleoptera: Coccinellidae: Scymninae: Scymnini
Diagnosis
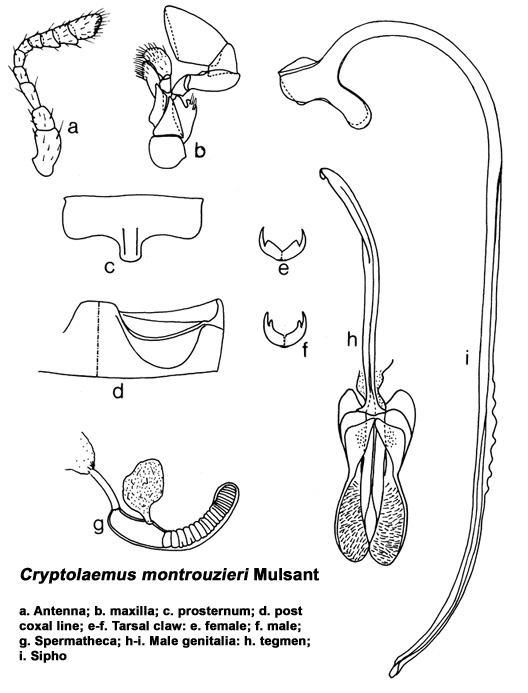
Length 3.8-4.6 mm, width 2.7-3.2 mm. Form elongate oval, convex. Head, mouth parts, antennae and pronotum reddish brown/orange yellow, scutellum dark brown to black, elytra shiny black with orange yellow or yellowish brown apices. Ventral side with prosternum orange yellow, meso- and
metasterna more or less black; abdomen orange yellow. Males with fore legs reddish brown, much paler than the other two pairs; abdomen with fifth and sixth segments emarginate, the latter more strongly so; tarsal claws bifid. In females, forelegs dark brown/black, of the same colour as other pairs of legs,
fifth and sixth abdominal segments truncate and arcuate, respectively; and tarsal claws appendiculate. Male genitalia and female spermatheca as illustrated. Larva covered with long tufts of white waxy filaments protruding from all directions, those along lateral margins usually longer than the rest.

 Ventral side of C. montrouzieri: Male (first pair of legs reddish brown / lighter than other pairs) and female (all pairs of legs black) Ventral side of C. montrouzieri: Male (first pair of legs reddish brown / lighter than other pairs) and female (all pairs of legs black)
Images





 Adult Adult
 Eggs laid on the ovisac of M. hirsutus (with smaller, orange eggs) Eggs laid on the ovisac of M. hirsutus (with smaller, orange eggs)



 Larva Larva

 Larvae and pupae Larvae and pupae
Distribution
Native of Australia and introduced into several countries such as India, New Zealand, United Kingdom, Italy, Spain, Greece, Hawaii, Brazil, Costa Rica, Puerto Rico, USA, Bermuda, Israel,
Indonesia, Philippines, Hong Kong, China, Egypt, South Africa, Trinidad, Grenada, Guyana, St. Kitts, and St. Lucia.
Hosts / Associated habitat
HEMIPTERA: Aleyrodidae: Aleurodicus dispersus Russell. Aphididae: Aphis craccivora Koch, Aphis gossypii Glover and Aphis nerii Boyer de Fonscolombe. Coccoidea: Aulacaspis citri Chen, Chrysomphalus pinnulifer (Maskell), Coccidohystrix
insolita (Green), Coccus hesperidum Linnaeus, Coccus longulus (Douglas), Coccus
pseudomagnoliarum (Kuwana), Coccus viridis (Green), Dactylopius ceylonicus (Green), Dactylopius confusus (Cockerell), Dactylopius opuntiae (Cockerell), Dactylopius tomentosus (Lamarck), Diaspidiotus sp., Drepanococcus chiton (Green), Dysmicoccus boninsis (Kuwana), Dysmicoccus brevipes (Cockerell), Eriococcus araucariae (Maskell), Eriococcus
coriaceus Maskell, Ferrisia virgata (Cockerell), Heliococcus summervillei Brookes, Icerya aegyptiaca (Douglas), Kilifia acuminata (Signoret), Lepidosaphes beckii (Newman), Maconellicoccus hirsutus (Green), Megapulvinaria maxima (Green), Nipaecoccus
aurilanatus (Maskell), Nipaecoccus filamentosus (Cockerell), Nipaecoccus nipae (Maskell), Orthezia tillandsiae Morrison, Parasaissetia nigra (Nietner), Phenacoccus gossypii Townsend & Cockerell, Philephedra tuberculosa Nakahara & Gill, Pinnaspis
aspidistrae (Signoret), Planococcus ficus (Signoret) (as Pseudococcus vitis (Neidielski)), Planococcus citri (Risso), Planococcus kenyae (Le Pelley), Planococcus kraunhiae (Kuwana), Planococcus lilacinus (Cockerell), Pseudococcus calceolariae (Maskell) (as P. fragilis Brain), Pseudococcus comstocki (Kuwana), Pseudococcus crotonis (Green), Pseudococcus hirsutus (Green), Pseudococcus longispinus (Targioni Tozzetti), Pseudococcus maritimus (Ehrhorn), Pseudococcus obscurus (Essig), Pseudococcus viburni (Signoret) (as P. affinis (Maskell)), Pseudococcus sp., Pulvinaria (as Chloropulvinaria) aurantii Cockerell, Pulvinaria (as Chloropulvinaria) floccifera (Westwood), Pulvinaria (as Saccharipulvinaria) iceryi (Signoret), Pulvinaria (as Chloropulvinaria) psidii Maskell, Puto barberi (Cockerell), Puto (as Ceroputo) sp., Rastrococcus iceryoides (Green), Saccharicoccus sacchari (Cockerell), Saissetia coffeae (Walker), Saissetia oleae (Olivier), Trionymus insularis (Ehrhorn). Psyllidae: Diaphorina citri Kuwayama. LEPIDOPTERA: Noctuidae: Spodoptera mauritia acronyctoides (Guenee). THYSANOPTERA: Scirtothrips citri (Moulton).
Predacious on mealybugs and soft scales infesting many horticultural and plantation crops, guava, mango,
grapevine, sapota, citrus, coffee, ornamental plants, mulberry, brinjal, Boehmeria sp., etc. Found congregating in large numbers on Araucaria pines in and around Bangalore.
Seasonal occurrence
Active almost throughout the year, less abundant during summer.
Natural enemies
Parasitoids: Hymenoptera: Encyrtidae: Cowperia indica (Kerrich), Cowperia (=Aminellus) sp.; Homalotylus flaminius (Dalman); Pteromalidae: Pseudocatolaccus sp.; Chalcididae: Spilochalcis porteri. Predators: Chrysopids, carabids and ants.
Remarks
This is a well-known species whose record as a successful introduced predator of mealybugs worldwide is rivalled only by Rodolia cardinalis. It is useful mainly in tropical climates. Since its first introduction into California in 1891 from Australia by Albert Koebele, and subsequently in 1893 in Hawaii, it has been imported into over 40 countries throughout the warm temperate and tropical regions of the world to combat mealybug pests (Clausen, 1978). Booth & Pope (1986) summarised the approximate dates and sources for the introduction of this predator throughout the world.
It is perhaps the first natural enemy deliberately introduced into India. It was originally introduced and released in India in 1898 on the Nilgiri Hills by H.O. Newport, an amateur entomologist, to check Coccus viridis and other mealybugs on coffee (Mayne, 1953) and apparently did not establish. Later, it was rediscovered in 1951, though probably it had established much earlier (Puttarudriah et al., 1952). Since then, it has become a common predator of mealybugs and soft scales infesting several horticultural and plantation crops and ornamental plants in South India. It is the only coccinellid widely mass
produced by several bioagent producers in India.
Mass production and field release
This species is commonly multiplied on mealybug-infested pumpkins in most of the Indian insectaries. Mealybugs such as P. citri, F. virgata and M. hirsutus, which produce ovisacs, have been used for mass production. The eggs of Sitotroga cerealella (Olivier) have been successfully used in Russia. However, females of C. montrouzieri oviposited on eggs of S. cerealella only in the presence of empty ovisacs of F. virgata (Mineo, 1967). At PDBC, a semi-synthetic diet has been developed for its mass production and a protocol has been standardised for its mass production on S. cerealella eggs.
 Grubs on mealybug-infested pumpkin
Grubs on mealybug-infested pumpkin
 Adults on mealybug-infested pumpkin
Adults on mealybug-infested pumpkinAdults are usually used for augmentative field releases as the larvae are cannibalistic. The release rate varies from 5-10 adults / mealybug-infested plant and depends on the crop and the mealybug species. For example, 1500 beetles / ha are released against Maconellicoccus hirsutus on grapevine. References
- Booth, R.G. & Pope, R.D. 1986. A review of the genus Cryptolaemus
(Coleoptera : Coccinellidae) with particular reference to the species resembling C. montrouzieri Mulsant. Bulletin of Entomological Research, 76, 701-717.
- CABI, 2002. Crop Protection Compendium. Global Module, 2nd edition (On CD-ROM). CAB International, Wallingford, UK.
- Gordon, R.D. 1985. The Coccinellidae of America, north of Mexico. Journal of the New York Entomological Society, 93, 1-912.
- Mani, M. & Krishnamoorthy, A. 1997. Australian ladybird beetle, Cryptolaemus montrouzieri. Madras Agricultural Journal 84: 237-249.
- Mayne, W.W. 1953. Cryptolaemus montrouzieri Mulsant in South India. Nature 172: 85.
- Mineo, G. 1967. Sul Cryptolaemus montrouzieri Muls (Osservazioni morphobiologische). Bollettino dell'Istituto di Entomologia Agraria e dell' Osservatorio di Fitopatologia di Palermo 6: 99-143.
- Moore, D. 1988. Agents used for biological control of mealybugs (Pseudococcidae). Biocontrol News and Information 9(4): 209-225.
- Pilipyuk, V.I., Bugaeva, L.N. & Baklanova, E.V. 1982. The possibility of rearing the predacious beetle, Cryptolaemus montrouzieri Muls. (Coleoptera, Coccinellidae) on eggs of the Angoumois grain moth. Entomologicheskoe Obozrenie, 61(1): 50-52.
- Puttarudriah, M. & Channabasavanna, G.P. 1953. Beneficial coccinellids of Mysore-I. Indian
Journal of Entomology 15: 87-96.
- Puttarudriah, M. & Channabasavanna, G.P. 1957. Notes on some predators of mealybugs (Coccidae,
Hemiptera). The Mysore Agricultural Journal 32: 4-19.
- Puttarudriah, M., Channabasavanna, G.P. & Krishnamurti, B. 1952. Discovery of Cryptolaemus montrouzieri Mulsant (Coccinellidae, Coleoptera, Insecta) in Bangalore, South India. Nature
169: 377.
|